Describe Electronegativity Trends in the Periodic Table
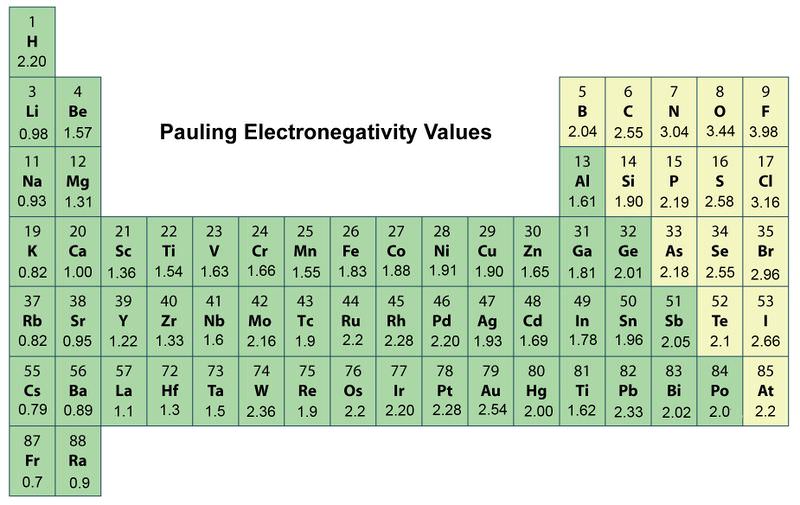
Fluorine the most electronegative element is assigned a value of 40 and values range down to caesium and francium which are the least electronegative at 07. It is often viewed on an.
So were asked to describe the Electra negativity turns in the periodic table.
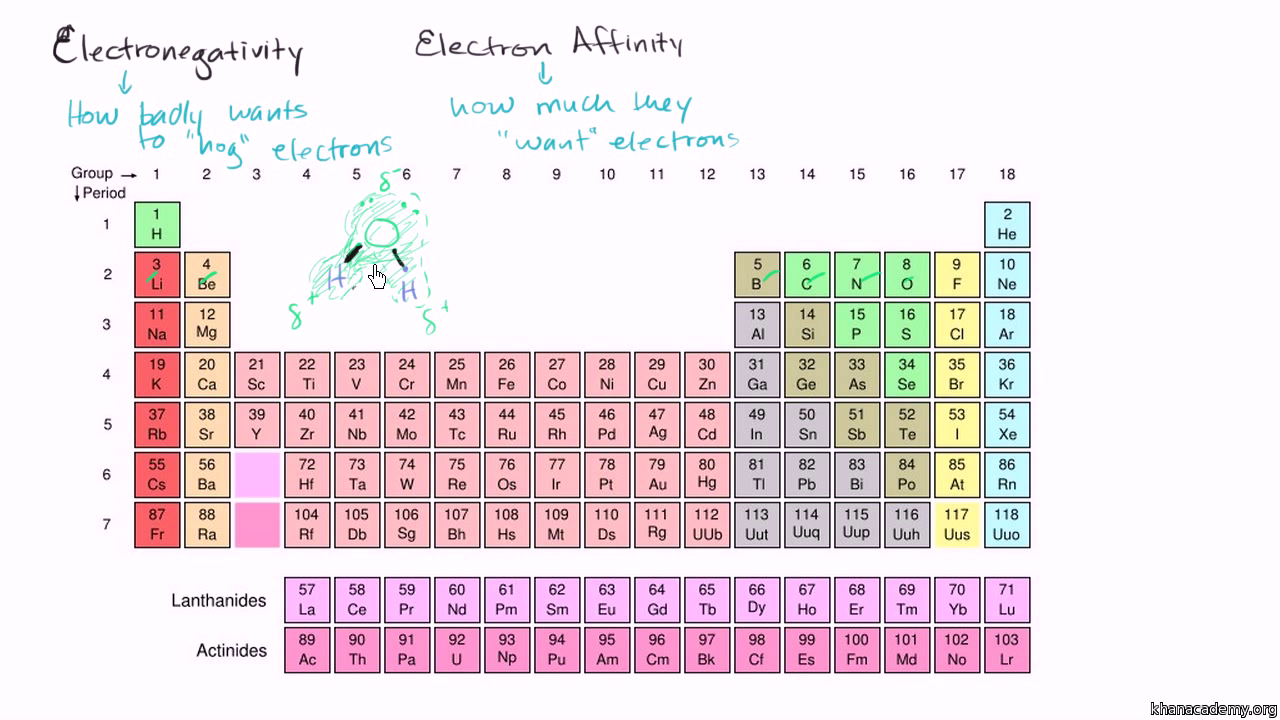
. Periodic table even though there are more protons in the elements at the bottom of the column. We identified it from honorable source. What best describes the trends in electronegativity on the periodic table.
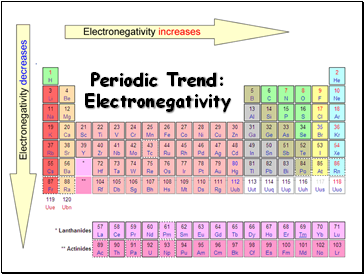
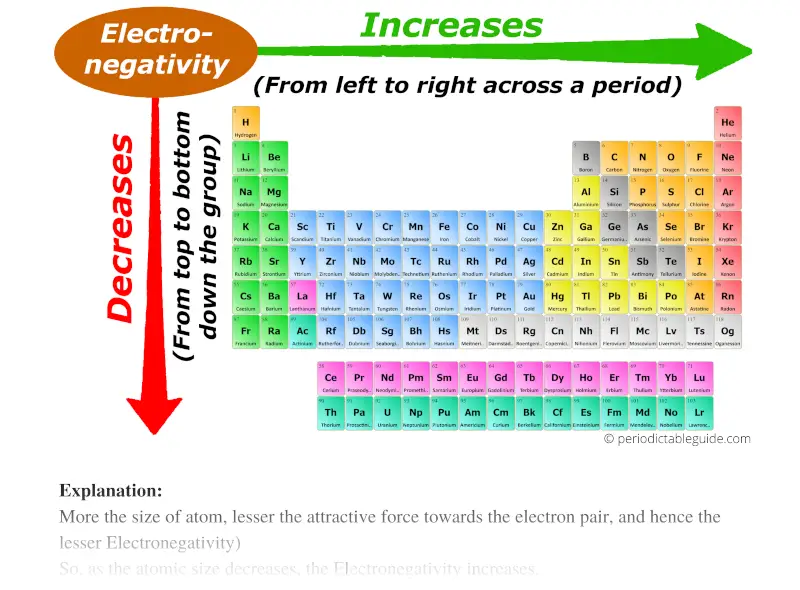
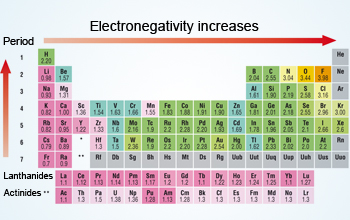
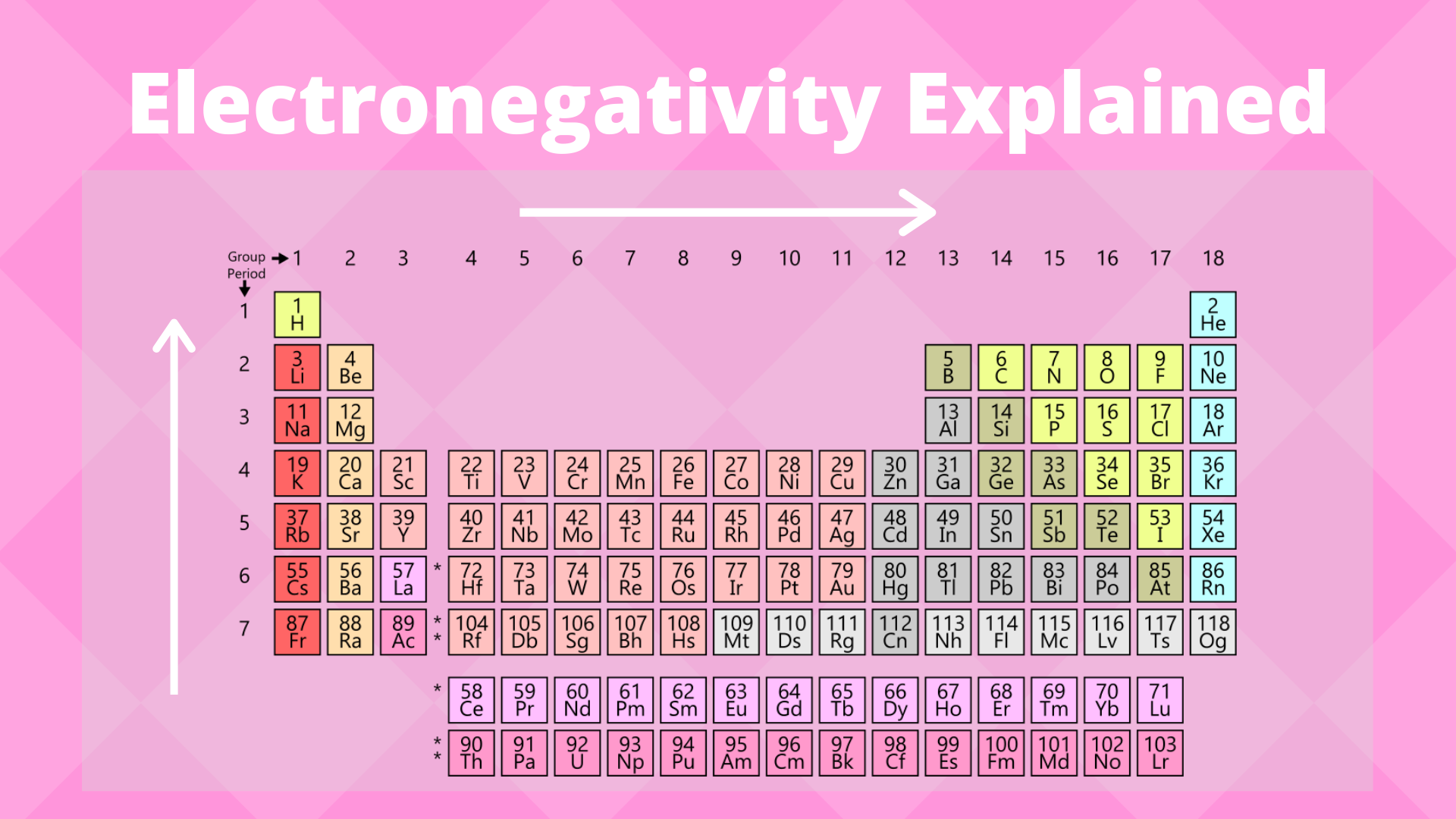
Electronegativity values generally increase from left to right across the periodic table. What best describes the trends in electronegativity on the periodic table. Electronegativity generally increases from left to right across a period and decreases down a column in the periodic table.
Fluorine is the most electronegative element. Electronegativity increases left to right and bottom to top. Here are a number of highest rated Periodic Table Electronegativities pictures on internet.
The electronegativity also increases up a group column of the periodic table. Electrostatic theory explains the trend in electronegativity in the Periodic Table in the Periodic Table both across a period and down a group. Electronegativity usually rises from left to right.
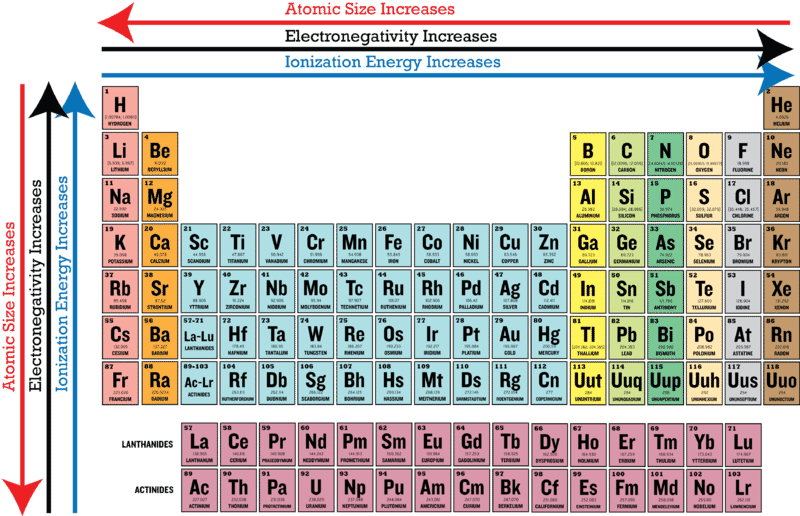
Describe the trends in atomic size ionization energy and electronegativity from left to right across a period in the periodic table. Lithium 10 and Fluorine 40 in period 2. There is an increase in the atomic number as we move down the group.
Electronegativity is the ability of an atom to pull electrons towards it. As mentioned the electronegativity trend refers to the way electronegativity values trend across the periodic table of the elements. 1 See answer Advertisement Advertisement hernandezalex1892 is waiting for your help.
Electronegativity tends to decrease down a group of the periodic table. It shows how an atom can swiftly form a chemical bond. Periodic Table And Electronegativity - 18 images - webelements periodic table silicon crystal structures electronegativity periodic table decoration items image periodic table trends surfguppy chemistry made easy electronegativity table periodic decoration items image.
The ability of an atom to attract electrons to itself in a chemical bond is called electronegativity. As you go across the elements in a period each element has an increased effective nuclear charge or Z eff attracting its outer shell electrons. Different elements have different electronegativities based on a number of factors such as size and number of protons neutrons and electrons.
When moving from left to right across the periodic table electronegativity increases with the exception being the noble gases. Electronegativity increases left to right and bottom to top. Electronegativity logo χ is a chemical property that explains the trend of an atom to attract a shared pair of electrons towards itselfAs you move from left to right across the periodic table electronegativity increases and as you move down the table electronegativity decreases.
Electronegativity is defined as an atoms ability to attract electrons towards it in a chemical bond. Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound. The Pauling scale is the most commonly used.
So if you look at the Bureau of Table you see that as a goal down the periodic table. 7 Describe the similarities in group trends on the line graph. Add your answer and earn points.
Electronegativities generally decrease from top to bottom of a group. In general electronegativity decreases as you move down a group in the periodic table this correlates. In this graph we have not shown argon as it does not react with elements to form bonds.
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Test your understanding with interactive textbook. There are several different ways of measuring it the most common being the Pauling scale.
Its submitted by doling out in the best field. Which group demonstrates the greatest deviation from the other trend shapes. Lithium 10 and Francium 07 in Group I.
It sees a decreasing trend when you move down a group. However there are exceptions to it at times. Electronegativity tends to increase as atomic size increases.
As we move across a period from left to right the nuclear charge increases and the atomic size decreases therefore the value of electronegativity increases across a period in the modern periodic table. For example the electronegativity trend across period 3 in the periodic table is depicted below. Electronegativity tends to decrease from left to right across a period of the periodic table.
Fluorine is the most electronegative element. Elements at the top of a column have greater electronegativities than elements at the bottom of a given column. When the Periodic Table of Elements was first devised by Dmitri Mendeleev.
When we move from left to right in a period of the modern periodic table electronegativity increases. We say yes this kind of Periodic Table Electronegativities graphic could possibly be the most trending topic afterward we allocation it in google improvement or facebook. Ahlukileoi and 28 more users found this answer helpful.
We can see this with the help of a graph showing the trend in electronegativity in period 3 from sodium to chlorine. 8 Evaluate the usefulness of a 3-D graph versus a line graph to compare values and analyze trends. How would you describe the periodic table of.
Electronegativity tends to decrease down a group of the periodic table. Well if were going down the number of Valence electron increase and the number of electrons surrounding an orbital increase because of this um like ability for a nucleus to be able to um cool electron Uh and holding. Periodic Table Trends.
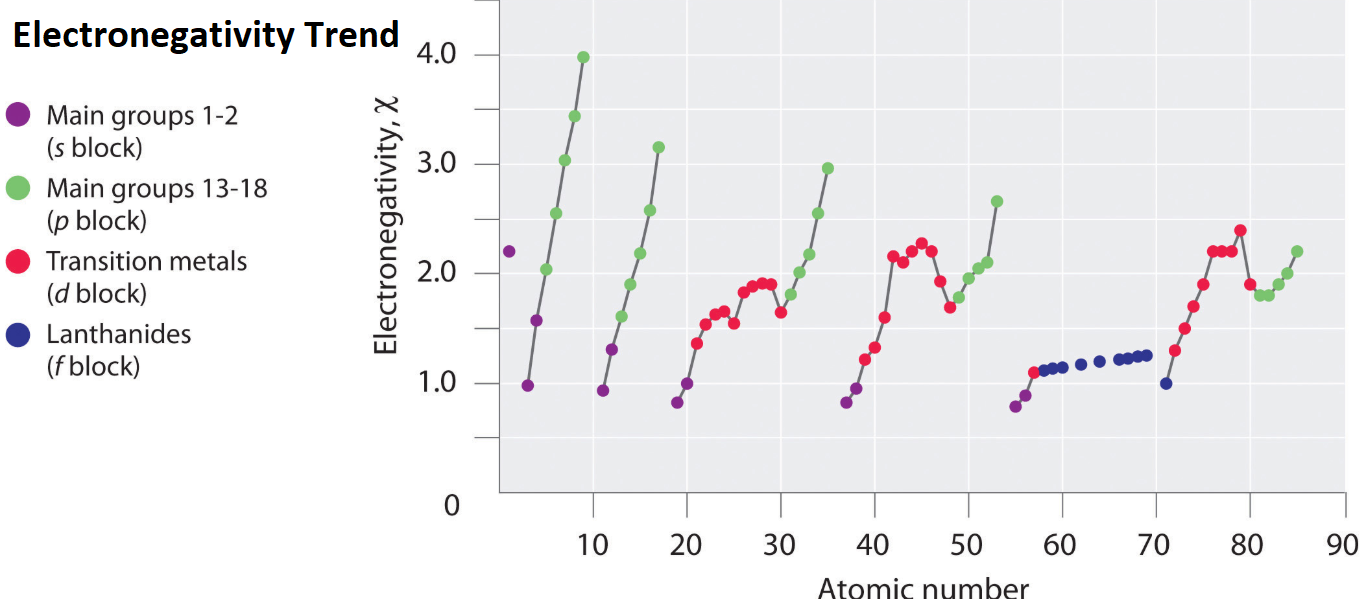
The trends for electronegativity is that the value increases across the periods rows of the periodic table. 6 Describe the 3-D periodic table bar chart of electronegativity values. Electronegativity Electronegativity relative ability of an atom to attract electrons in a chemical bond.
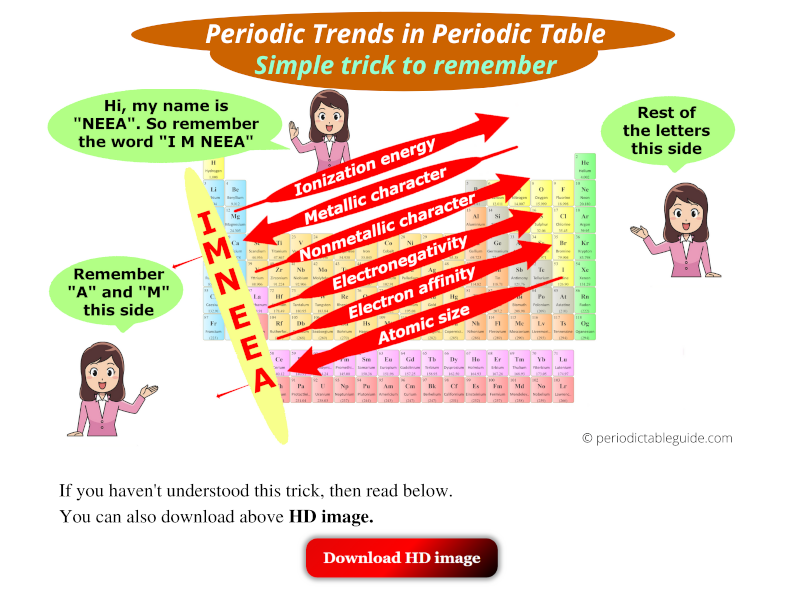
Metals tend to have lower electronegativities than nonmetals. Metals tend to have lower electronegativities than nonmetals. The overall trend for electronegativity in the periodic table is diagonal from the lower left corner to the upper right corner.
Trends in electronegativity across a period. Effective nuclear charge is the positive charge that.
Periodic Trends Presentation Chemistry
All Periodic Trends In Periodic Table Explained With Image

What Is Electronegativity Trends Chart Periodic Table Chemtalk

Periodic Trends Electronegativity Chemistry For Non Majors
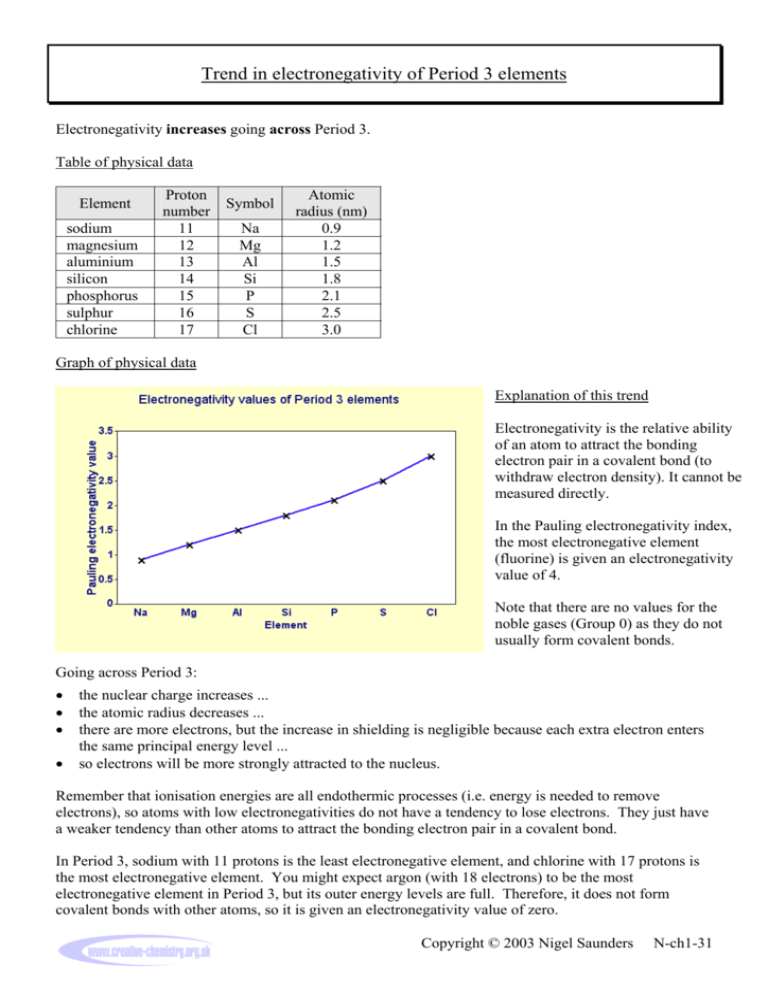
Trend In Electronegativity Of Period 3 Elements
Periodic Trends In Electronegativity Ck 12 Foundation

Periodic Trends Definition And Properties

Ketan Periodic Trends Explained Again But Examples Are Given Chemistry Physical Science Ionization Energy

Electronegativity Examples Trends Video Lesson Transcript Study Com

Periodic Trends In Electronegativity Youtube

Electronegativity Video Periodic Trends Khan Academy

Periodic Trends Made Easy Chemtalk
All Periodic Trends In Periodic Table Explained With Image

Electronegativity Trends Of The Periodic Table Bulb
Periodic Trends In Electronegativity Ck 12 Foundation
What Is Electronegativity Trend Example Education Career

What Is Electronegativity Trends Chart Periodic Table Chemtalk
Comments
Post a Comment